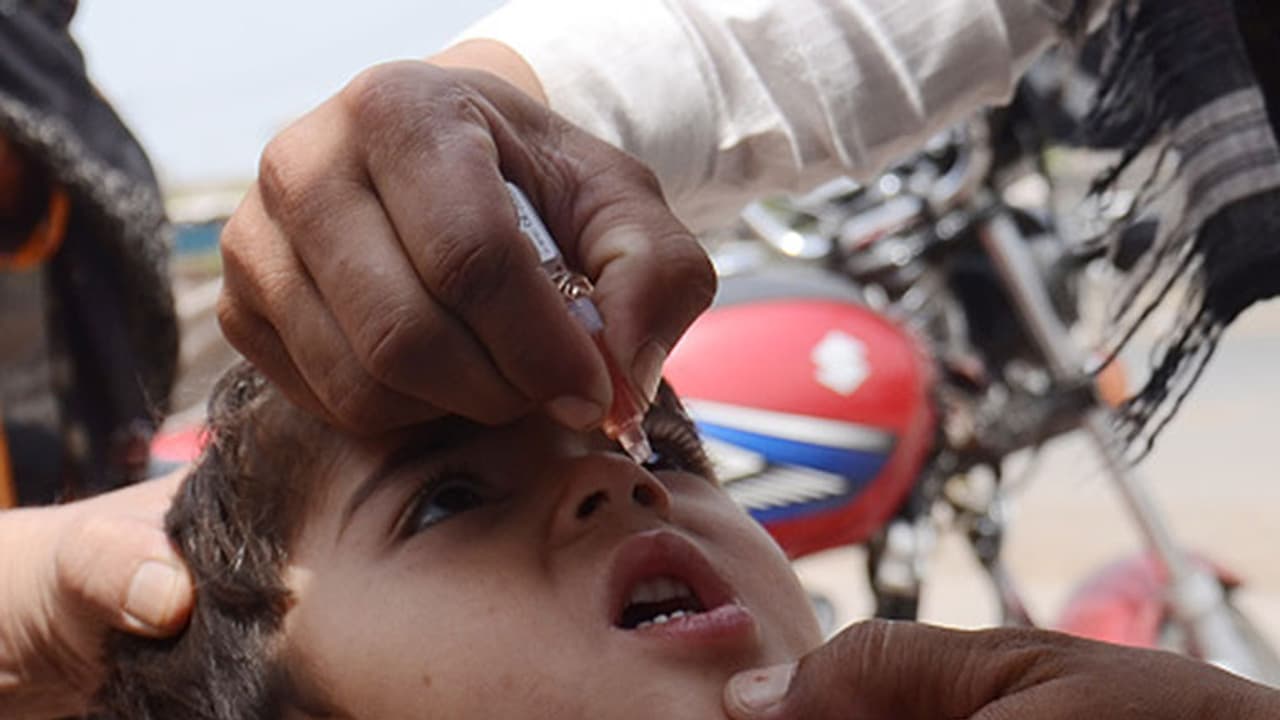
A couple of days back, some batches of oral polio vaccine manufactured by a Ghaziabad-based pharmaceutical company were detected with traces of Polio Virus Type 2
After traces of Polio Virus Type 2 were found in some in some batches of oral polio vaccine, the Union health ministry has stepped up the surveillance in states like Uttar Pradesh, Maharashtra and Telangana.
In a statement, the ministry said it has noted the matter and a probe has been ordered. A constant vigil is being kept on the shedding of the polio vaccine virus in these areas, officials said.
It is to be noted that a couple of days back, some batches of oral polio vaccine manufactured by a Ghaziabad-based pharmaceutical company were detected with traces of Polio Virus Type 2.
Sources said no sample has tested positive for wild polio virus in sewage or acute flaccid paralysis cases since 2011. “The country remains polio-free and this status has been maintained for more than seven years since the last wild polio virus case in the country was reported in January 2011. No child has been infected with wild polio virus as reported in some sections of the media," the ministry statement said.
The Type 2 polio vaccine virus traces, which have been found in bivalent Oral Polio Vaccine (bOPV) vials, is a weakened polio virus and does not cause paralysis. It was also earlier used in vaccines till April 2016.
The Health Ministry has asked the surveillance teams in the three states to track all those children who have been given the vaccine and keep a close watch for any symptoms. To enhance immunity against type 2 polio virus further, special mop up rounds for administering IPV are being conducted in the specified areas to reach out to such children who may have missed it.
This would provide immunity to all the children against all the three types of polio virus including type 2, the statement stated. After the contamination came to light, the use of all the vaccine supplied by manufacturer was immediately stopped in the country till investigation is completed. "Some legal samples of bOPV were immediately sent for testing to the Central Drug Laboratory in Kasauli, which confirmed the previous report of presence of traces of P2 polio vaccine virus," the statement said.
The Drugs Controller General of India immediately lodged a case and issued notice to the company asking it to stop manufacturing and supplying till further orders. The MD of the company was immediately arrested, the statement said.
The statement added that the detection of type 2 vaccine virus indicates that a very robust polio surveillance jointly managed by the Ministry of Health and Family Welfare and the WHO is still maintained even after seven years have elapsed since the last wild poliovirus case in the country was reported.
Last time, a type 2 wild polio virus case was detected in 1999. A team of health ministry, DCGI, ICMR and the WHO is continuously monitoring the situation. "The Government of India is committed as always to ensure that all vaccines used in the programme are safe and effective," the statement stated.